UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
|
|
|
|
|
|
|
|
(Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
|
|
|
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 7.01. Regulation FD.
Finch Therapeutics Group, Inc. (the “Company”) from time to time presents and/or distributes to the investment community, at various industry and other conferences, slide presentations to provide updates and summaries of its business. On September 13, 2021, the Company posted an updated corporate presentation to its website. The corporate presentation is available under the “Events & Presentations” tab in the “Investors & News” section of the Company’s website, located at www.finchtherapeutics.com.
The information in this Item 7.01 of this Current Report on Form 8-K shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section, nor shall such information be deemed incorporated by reference in any other filing with the Securities and Exchange Commission made by the Company, except as shall be expressly set forth by specific reference in such a filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
|
Exhibit No. |
|
Description |
|
|
|
|
|
99.1 |
|
|
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
1
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
FINCH THERAPEUTICS GROUP, INC. |
|
|
|
|
|
|
|
Date: September 13, 2021 |
|
By: |
/s/ Mark Smith |
|
|
|
|
Mark Smith, Ph.D. |
|
|
|
|
Chief Executive Officer |
2

Harnessing the Genomic Revolution & Machine Learning to Pioneer Microbiome Therapeutics CORPORATE PRESENTATION | SEPTEMBER 2021 Exhibit 99.1

Forward-Looking Statements Statements contained in this presentation regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Words such as "anticipates," "believes," "expects," "intends," “plans,” “potential,” "projects,” “would” and "future" or similar expressions are intended to identify forward-looking statements. These forward-looking statements include, but are not limited to, statements regarding: the growth, strategy, initiation, timing, progress and results of the Company’s current and future research and development programs, preclinical studies and clinical trials and related preparatory work and the period during which the results of such trials will become available, including specifically the initiation and conduct of a Phase 3 trial in recurrent C. difficile and Phase 1 trials in autism and chronic hepatitis B and the timing of data readouts from those trials; the Company’s and its collaborators’ ability to obtain regulatory approval of CP101, FIN-211, TAK-524, FIN-525 and any other current and future product candidates that it develops; the Company’s ability to develop additional product candidates; its expectations regarding the potential market size and the rate and degree of market acceptance for any product candidates that it develops; the therapeutic value and commercial potential of candidates developed using its Human-First Discovery platform; the completion of its commercial manufacturing facility; and the Company’s expected cash runway. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. These risks and uncertainties include, among others: the Company’s limited operating history and historical losses; the Company’s ability to raise additional funding to complete the development and any commercialization of its product candidates; the Company’s dependence on the success of its lead product candidate, CP101; the possibility that the Company may be delayed in initiating, enrolling or completing any clinical trials; results of clinical trials may not be sufficient to satisfy regulatory authorities to approve the Company’s product candidates in their targeted or other indications (or such authorities may request additional trials or additional information); results of clinical trials may not be indicative of final or future results from later stage or larger clinical trials (or in broader patient populations once the product is approved for use by regulatory agencies) or may not be favorable or may not support further development; the Company’s product candidates, including CP101, may not generate the benefits to patients that are anticipated; anticipated regulatory approvals may be delayed or refused; competition from third parties that are developing products for similar uses; the Company’s ability to maintain patent and other intellectual property protection and the possibility that the Company’s intellectual property rights may be infringed, invalid or unenforceable or will be threatened by third parties; the Company’s ability to qualify and scale its manufacturing capabilities in anticipation of commencement of multiple global clinical trials; the Company’s lack of experience in selling, marketing and distributing its product candidates; the Company’s dependence on third parties in connection with manufacturing, clinical trials and preclinical studies; and risks relating to the impact and duration of the COVID-19 pandemic on the Company’s business. These and other risks are described more fully in the Company’s filings with the Securities and Exchange Commission (“SEC”), including the section titled “Risk Factors” in the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 10, 2021, as well as discussions of potential risks, uncertainties, and other important factors in the Company’s other filings with the SEC. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Except to the extent required by law, the Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. Finally, while the Company believes its own internal research is reliable, such research has not been verified by any independent source. Human-First Discovery® is a registered trademark of the Company. 2

Accomplished leadership team with experience in innovation, development, and commercial execution 3

The microbiome is an untapped target for therapeutic intervention 4 Sources: Tierney Cell Host Microbe 2019 ~20K human genes >20M microbial genes Immune modulation Humans carry 1000-fold more microbial genes than host genes The microbiome is an organ system fundamental to human health Enabled by genomics and data science, Finch is pioneering microbiome therapeutics Metabolic function Neurologic regulation

Differentiated discovery process, with proof-of-concept clinical data leveraged to guide product design and de-risk development Uniquely positioned to harness full diversity and potential of the microbiome across diverse therapeutic areas Leading machine learning-based platform recognized by Takeda partnership Data-rich period ahead, with multiple programs advancing towards the clinic Positive pivotal data with lead asset provides foundation for future growth Investment Highlights 5

Growing body of clinical evidence across diverse therapeutic areas 6 Source: Clinicaltrials.gov Oncology Hepatology Metabolic Neuropsychiatric Infectious disease Gastrointestinal 314 Other

Our Human-First Discovery platform enables capital efficient de-risking 7 Enabled by: Machine learning engine Enabled by: Proprietary access to data 2. Data-Mining for Mechanistic Insights 1. Clinical Proof-of-Concept (3rd party data) 3. Product Development Enabled by: Platform to target full microbiome Program launch & capital commitment

Finch is the only company with both complete and targeted approaches for developing microbiome therapeutics 8 DONOR-INDEPENDENT DONOR-DERIVED Complete Consortia Enriched Consortia Targeted Consortia Delivers complete microbial community to restore broad community function Delivers selected microbes to target specific biological pathways Hybrid approach to restore broad community function and target specific pathways

Finch is advancing a diverse portfolio 9 Takeda to lead development

CP101 for Recurrent C. difficile Infection (CDI)

CP101, an orally administered, purified microbiome product candidate delivers a complete microbial community Lyophilization technology optimized to preserve entire community, enabling use across multiple indications 11 Spores (25-50 taxa) TLR agonists Chronic HBV 2° bile acid production Recurrent CDI Proprietary anti-inflammatory metabolite IBD Oxytocin induction Autism Non-Spores (500-1,000 taxa) Lyophilization & Encapsulation 2. Harvest, Purification, & Preservation 3. Lyophilization & Encapsulation 1. Healthy Donor Sourcing & Qualification Complete consortia composition provides potential for broad label expansion All Bacterial Taxa Efficient, scalable manufacturing enabled by molecular screening of donors

Recurrent CDI is an enormous human and economic burden 12 Sources: Zhang BMC Infect Dis 2016; Dehlholm-Lambertsen Ther Adv Gastroenter 2019 (1 EUR = 1.1482 USD); Desai BMC Infect Dis 2016; CDC Antibiotic Resistance Threat Report 2019 CDI CP101 Complete Consortia delivers full microbiome community

CP101 is positioned to serve a large population in recurrent CDI 13 Sources: Desai BMC Infect Dis 2016 38K 46K 115K 461K ≥3 Recurrences 19% of recurrent CDI cases 2nd Recurrence 23% of recurrent CDI cases 1st Recurrence 58% of recurrent CDI cases Primary Cases 199K total recurrent CDI cases CDI in the US CDI CP101 target population
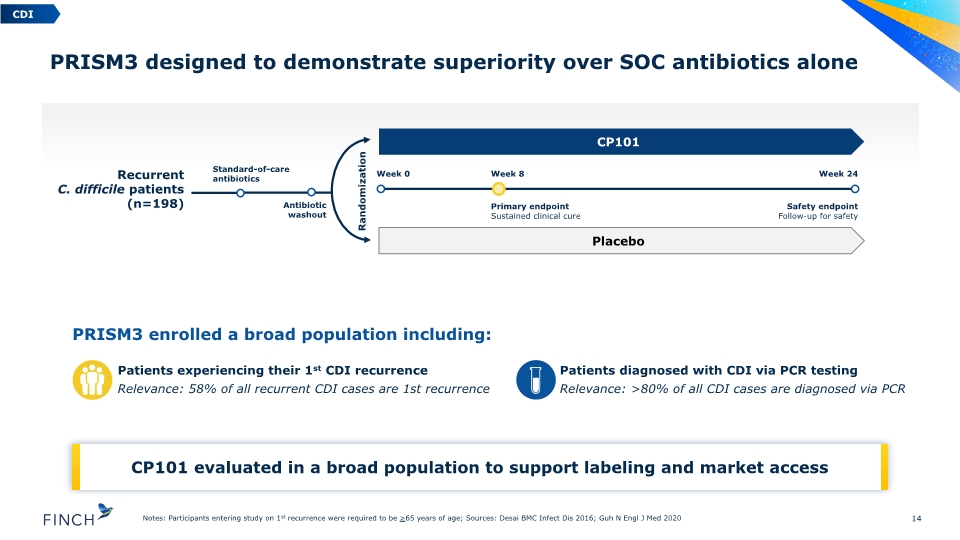
PRISM3 enrolled a broad population including: PRISM3 designed to demonstrate superiority over SOC antibiotics alone 14 Notes: Participants entering study on 1st recurrence were required to be >65 years of age; Sources: Desai BMC Infect Dis 2016; Guh N Engl J Med 2020 Recurrent C. difficile patients (n=198) Standard-of-care antibiotics Antibiotic washout CP101 Placebo Week 0 Week 8 Primary endpoint Sustained clinical cure Week 24 Safety endpoint Follow-up for safety Randomization Patients diagnosed with CDI via PCR testing Relevance: >80% of all CDI cases are diagnosed via PCR Patients experiencing their 1st CDI recurrence Relevance: 58% of all recurrent CDI cases are 1st recurrence CDI

CP101 achieved its primary efficacy endpoint and demonstrated a favorable tolerability profile in PRISM3 15 Primary efficacy analysis: Sustained clinical cure through Week 8 Recurrence determined by blinded adjudication board p=0.0488 CDI SAEs: Serious adverse events Log-rank test p=0.0139 Placebo CP101 Days Post Randomization CP101 met its primary efficacy endpoint in a broad population, with no treatment-related SAEs in the CP101 arm Percentage of participants with CDI recurrence (%) Recurrence determined by blinded adjudication board

CP101 engrafts new species, altering the structure of the microbiome 16 Source: CP101 Phase 2 study (PRISM3) p<0.0001 Number of engrafted CP101-associated taxa CDI

Strong relationship between CP101 engraftment and clinical outcomes in PRISM3 17 Source: CP101 Phase 2 study (PRISM3) Sustained clinical cure by engraftment group p<0.001 Number of engrafted CP101-associated taxa CDI

Topline readout from Phase 3 trial of CP101 in recurrent CDI expected in H1 2023 18 Recurrent CDI patients (n~300) Standard-of-care antibiotics Antibiotic washout CP101 Placebo Week 0 Week 8 Primary endpoint Week 24 Safety endpoint Randomization Key Features Extension of antibiotic washout period to enhance engraftment Sample size increased to enhance power Global study to support marketing authorizations outside the US CDI

Additional CP101 data reading out in 2021 19 Week 0 Week 8 Efficacy and safety evaluation Week 24 Long-term follow-up CP101 administration Recurrent CDI patients (n >130) CP101 Standard-of-care antibiotics Antibiotic washout CDI Additional clinical data with CP101 will contribute to overall safety database

CP101 positioned to be market leader in recurrent CDI 20 CDI Efficient, scalable manufacturing enabled by molecular rather than chemical pathogen exclusion Convenient, one-time oral administration Achieved primary endpoint in a broad population, positioning CP101 to serve a significant patient population: All stages of recurrent CDI All test methods for CDI diagnosis Complete consortia composition provides potential for broad label expansion Fast Track and Breakthrough Therapy designations for prevention of recurrent CDI

TAK-524 & FIN-525 for Inflammatory Bowel Disease (IBD)

Finch & Takeda working together to develop new therapeutics for IBD 22 Sources: Dahlhamer MMWR 2016; Crohn’s and Colitis Foundation: Facts About IBD 2014; Bernstein Inflamm Bowel Dis 2010 IBD TAK-524 & FIN-525 Targeted Consortia

Finch’s machine learning platform enables identification and isolation of promising targets from clinical data TAK-524 illustrates the power of Finch’s platform for the development of Targeted Consortia 23 Sources: Rossen Gastroenterology 2015; Moayyedi Gastroenterology 2015; Paramsothy Lancet 2017; Costello JAMA 2017; Sandborn Gastroenterology 2012 Remission rates for induction in active UC (%) Variation in effectiveness across donors supports Targeted Consortia approach IBD Takeda recently accelerated its leadership role in the development of the TAK-524 ulcerative colitis program

Finch’s combination of proprietary data and machine learning capabilities enable differentiated Targeted Consortia 24 2. Proprietary Algorithms Uncover Strain-Level Hits Reverse Translation Narrows Search Space 3. Strain Isolation from Effective Donors 4. Mechanism of Action Data Generation Depletion of microbes in patients with target condition, compared to healthy controls Abundance change in FMT responders Top targets identified High throughput molecular screens Human cells/tissue In vivo models Phylogenetic analysis to identify strain-level signals Isolation of specific strains from donor samples that demonstrated promising results in the clinic Finch’s platform brings the power of AI to microbiome therapeutic development

FIN-211 for Autism Spectrum Disorder (ASD)

ASD is a significant unmet need linked to the gut-brain axis 26 Sources: Chaidez J Autism Dev Disord 2014; Cao Shanghai Arch Psychiatry 2013; CDC Data and Statistics on ASD 2019; Leigh J Autism Dev Disord 2015 Finch plans to initially focus on the subset of the ASD population suffering from significant GI symptoms ASD Enriched Consortia Complete Consortia addresses community level dysbiosis Targeted Consortia ensure key mechanisms are consistently engaged FIN-211

Multiple lines of evidence point to the role of the microbiome in ASD 27 Sources: Ding J Autism Dev 2017; Zhang JAMA Netw Open 2019; Bittker Neuropsychiatr Dis Treat 2018; Modahl Biol Psychiatry 1998; Sgritta Neuron 2019; Needham Biol Psychiatry 2020; Hsiao Cell 2013; Antonini Front Immunol 2019; Kang Microbiome 2017; Kang Sci Rep 2019; Zhao Gastrointest Endosc 2019 (DDW Abstract); Ward Open Forum Infect Dis 2016 (ID Week Abstract); Li Zhonghua Wei Chang Wai Ke Za Zhi 2019 Distinct microbiome composition among individuals with ASD Early life events that impact the microbiome are associated with increased risk of ASD Cesarean section: 33% higher ASD risk Reduced breast feeding: 93% - 107% higher ASD risk Antibiotics: 144% - 264% higher ASD risk Oxytocin: Depleted levels of oxytocin in those with ASD Key, non-spore microbes induce oxytocin production Gut barrier: Impaired gut barrier integrity and translocation of behavior-influencing metabolites (e.g. 4-EPS) Microbiome enhances gut barrier integrity Multiple FMT studies show improvements in both GI and behavioral endpoints 1. Dysbiosis 2. Mechanistic insights 3. PoC FMT clinical studies ASD

Open label data shows improvements in both GI and behavioral symptoms following microbiota transplantation 28 Sources: Kang Microbiome 2017; Kang Sci Rep 2019 Children with ASD (n=18) Week 0 Week 8 Daily maintenance FMT doses 8 weeks post treatment Gastrointestinal Symptom Rating Scale (GSRS) and Childhood Autism Rating Scale (CARS) assessed at 8-weeks and 2 years post treatment 2 years post treatment High dose FMT Moderate ASD Cutoff Severe Mild Each dot represents an individual child ASD
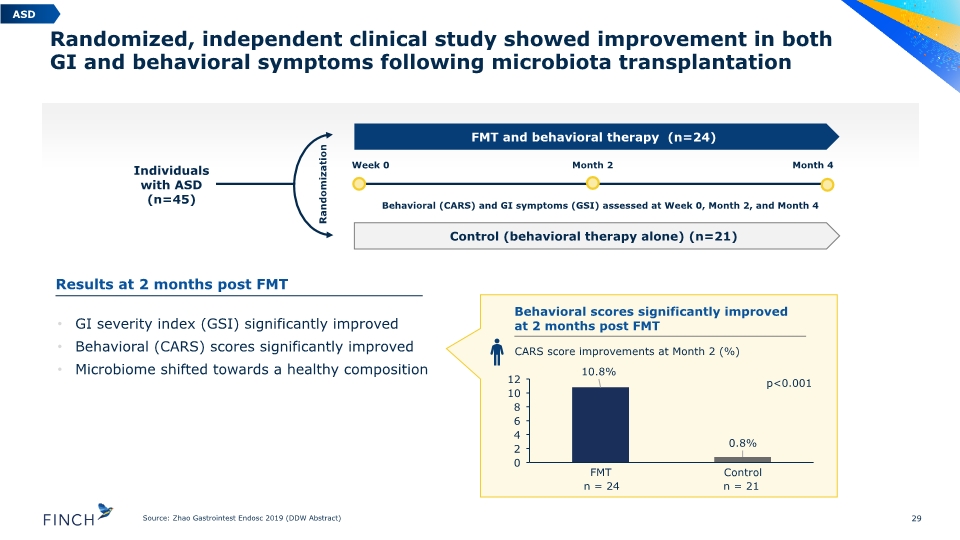
Randomized, independent clinical study showed improvement in both GI and behavioral symptoms following microbiota transplantation 29 Source: Zhao Gastrointest Endosc 2019 (DDW Abstract) Individuals with ASD (n=45) FMT and behavioral therapy (n=24) Control (behavioral therapy alone) (n=21) Week 0 Month 2 Behavioral (CARS) and GI symptoms (GSI) assessed at Week 0, Month 2, and Month 4 Month 4 Randomization n = 24 n = 21 p<0.001 CARS score improvements at Month 2 (%) GI severity index (GSI) significantly improved Behavioral (CARS) scores significantly improved Microbiome shifted towards a healthy composition ASD

Preclinical data show oxytocin-dependent behavioral improvements with microbiome therapy 30 Sources: Sgritta Neuron 2019 Interaction (s) Oxytocin levels Interaction (s) Interaction (s) ASD

FIN-211 is designed to address both the gastrointestinal (GI) and behavioral symptoms of ASD 31 ASD Pre-IND FDA feedback yielded two key insights: 1. FIN-211 may proceed directly to children with ASD 2. Demonstrating benefit for either GI or behavioral symptoms could support a BLA

Phase 1b AUSPIRE trial will evaluate multiple dosing regimens of FIN-211 in children with ASD and GI symptoms Children with ASD-GI (n~20) 32 ASD Vancomycin Pre-Treatment Low Dose No Vancomycin Pre-Treatment High Dose 2 weeks of FIN-211 dosing 8 weeks of FIN-211 dosing at highest tolerated dose from AUSPIRE Part A Children with ASD-GI (n~24) NEW

CP101 for Chronic Hepatitis B Virus (HBV)

Chronic HBV is the first label expansion opportunity for CP101 34 Sources: WHO Global Hepatitis Report 2017; CDC Hepatitis B: The Pink Book; Committee on a National Strategy for the Elimination of Hepatitis B and C; Hepatitis B Foundation; Van der Hilst Med Care Res Rev 2009 Clinical data support the role of microbiome in chronic HBV HBV CP101 Complete Consortia delivers full microbiome community
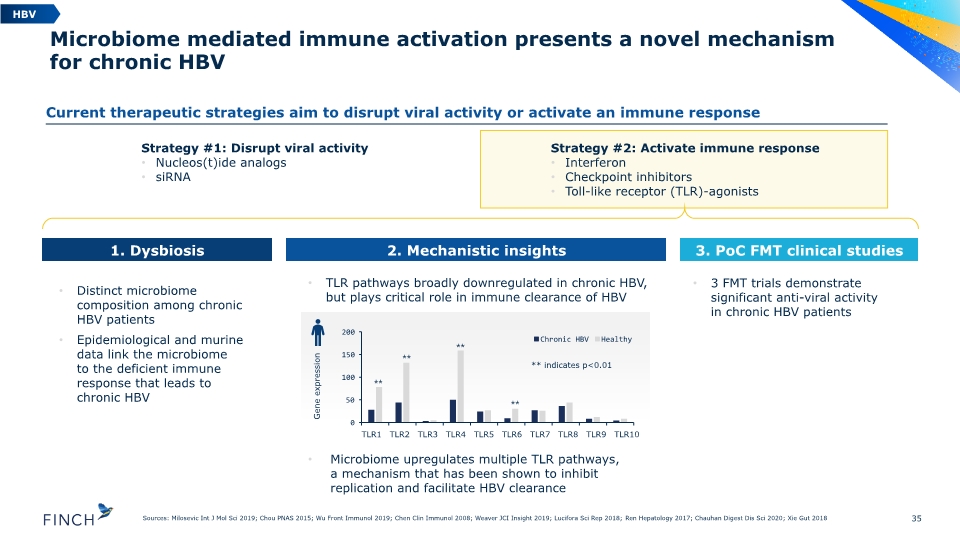
TLR pathways broadly downregulated in chronic HBV, but plays critical role in immune clearance of HBV Microbiome upregulates multiple TLR pathways, a mechanism that has been shown to inhibit replication and facilitate HBV clearance 35 Sources: Milosevic Int J Mol Sci 2019; Chou PNAS 2015; Wu Front Immunol 2019; Chen Clin Immunol 2008; Weaver JCI Insight 2019; Lucifora Sci Rep 2018; Ren Hepatology 2017; Chauhan Digest Dis Sci 2020; Xie Gut 2018 Distinct microbiome composition among chronic HBV patients Epidemiological and murine data link the microbiome to the deficient immune response that leads to chronic HBV 3 FMT trials demonstrate significant anti-viral activity in chronic HBV patients Microbiome mediated immune activation presents a novel mechanism for chronic HBV Strategy #1: Disrupt viral activity Nucleos(t)ide analogs siRNA Strategy #2: Activate immune response Interferon Checkpoint inhibitors Toll-like receptor (TLR)-agonists Gene expression ** indicates p<0.01 ⁎⁎ ⁎⁎ ⁎⁎ ⁎⁎ HBV 1. Dysbiosis 2. Mechanistic insights 3. PoC FMT clinical studies

Studies show that a functional microbiome enables HBV clearance 36 Sources: Chou PNAS 2015; Wu Front Immunol 2019 Mechanism tied to immune activation HBV

CP101 is positioned to address two important clinical objectives 37 Sources: Ott BMC Infect Dis 2012; Yang N Engl J Med 2002 Objective #1: Achieve HBeAg clearance among HBeAg positive patients Objective #2: Reduce HBsAg in HBeAg negative patients 20% HBeAg positive 80% HBeAg negative Chronic HBV population Cumulative incidence of liver cancer (%) Year HBsAg positive, HBeAg positive HBsAg positive, HBeAg negative HBsAg negative, HBeAg negative HBV

Multiple clinical studies with microbiota transplantation show improved HBV pathology 38 Sources: Ren Hepatology 2017; Chauhan Digest Dis Sci 2020; Xie Gut 2018 6-month HBV DNA reduction (Log10 ) % change in HBsAg titer Antiviral therapy alone FMT and antiviral therapy Antiviral therapy alone FMT and antiviral therapy Antiviral therapy alone FMT and antiviral therapy HBeAg titer (log10[S/CO]) Chauhan 2020 Ren 2017 Xie 2018 p=0.0002 Trial 1: HBeAg positive Trial 2: HBeAg positive Trial 3: HBeAg negative HBeAg clearance HBV
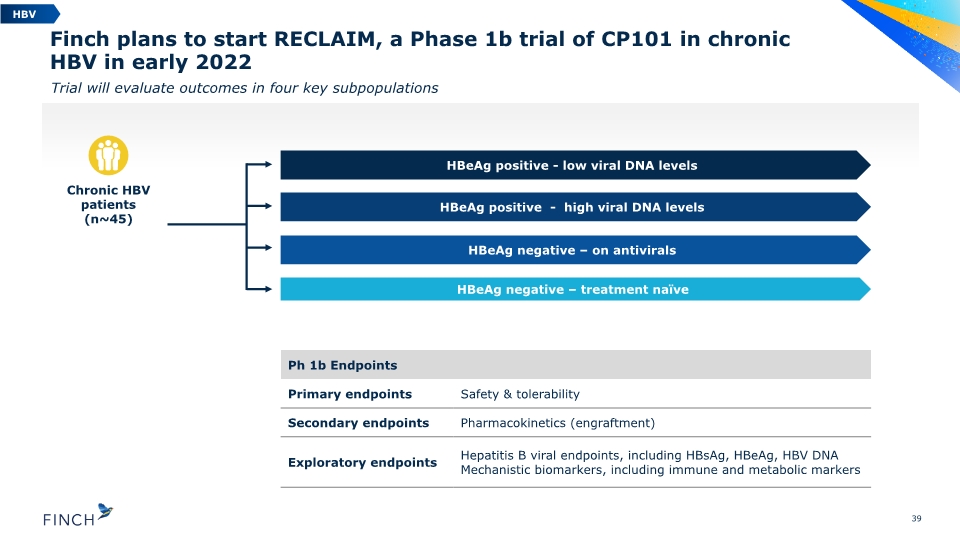
Finch plans to start RECLAIM, a Phase 1b trial of CP101 in chronic HBV in early 2022 Trial will evaluate outcomes in four key subpopulations 39 HBeAg negative – on antivirals HBeAg positive - low viral DNA levels Chronic HBV patients (n~45) HBV HBeAg negative – treatment naïve HBeAg positive - high viral DNA levels

Anticipated Milestones

Finch positioned to continue momentum 41 *As of 6/30/2021, unaudited cash and cash equivalents of $168 million 2020 Positive topline data from PRISM3 trial of CP101 in recurrent CDI Completed $90M financing Completed pre-IND meeting for ASD FDA confirmation of pivotal nature of PRISM3 and path to BLA Completed upsized $130.8M IPO Takeda accelerated leadership role in TAK-524 ulcerative colitis program Initiate PRISM4 in recurrent CDI Readout from PRISM-EXT in recurrent CDI Initiate Phase 1b trial in ASD Complete commercial manufacturing facility Initiate Phase 1b trial in chronic HBV Initial safety review from Phase 1b in chronic HBV Readout from Phase 1b trial in ASD Readout from Phase 1b trial in chronic HBV Anticipated milestones 2021 2022 Strong balance sheet with anticipated runway into mid-2023*

Harnessing the microbiome to transform patients’ lives
